Saisei Mirai Featured in Science Featured – Telomere Research Highlighted!
Dr. Inui’s Research on Telomere Length Featured in Science Featured We are pleased to share that Dr. Toshio Inui, CEO of Saisei Mirai Clinics Group, has been featured in the…
Saisei Medical Conference 2025
📍 Osaka International Convention Centre, 📅 Saturday, November 8, 2025 | 10:00 AM – 5:00 PM A Global Gathering in Osaka This November, Saisei Mirai Clinic proudly presents the Saisei Medical…
A New Era of Dietary Rejuvenation: Phase 2 Clinical Trial of Dietary MAF in Japan
The desire to live longer—and live well—is universal. Yet as we age, our cells and genes gradually change, revealing measurable signs of biological aging.One emerging approach attracting scientific attention is MAF…
MAF Boosts Longevity Protein α-Klotho!
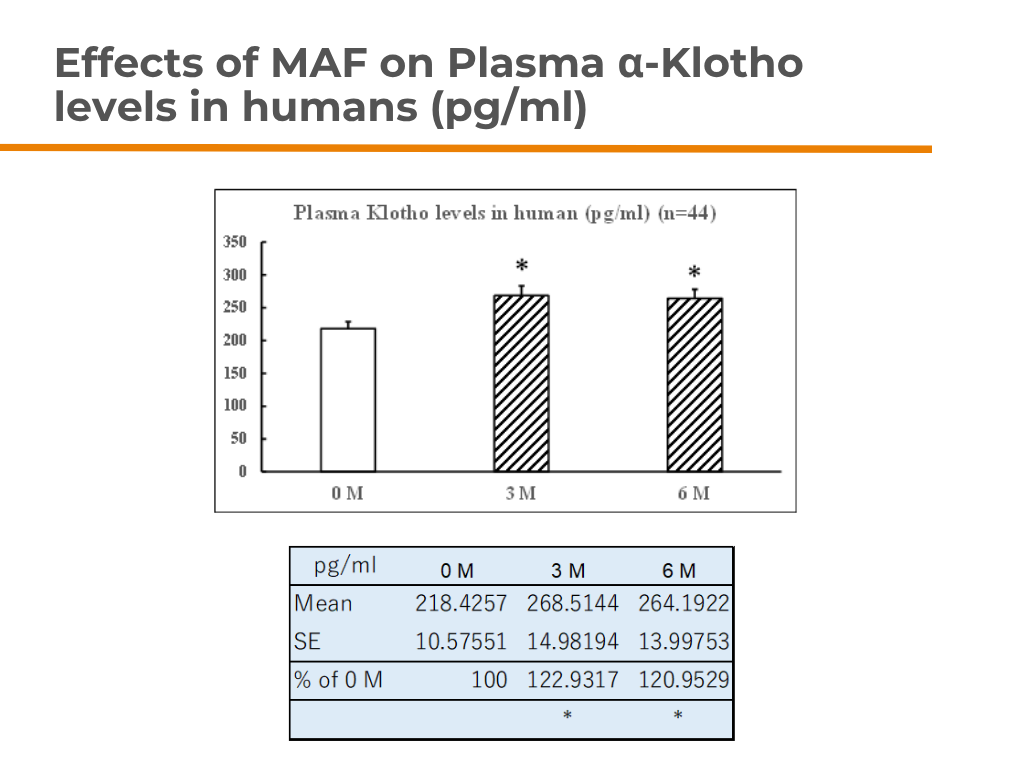
A groundbreaking experiment by Dr. Katuura (formerly Kagoshima University, now at Saisei Mirai) showed that dietary MAF significantly increased α-Klotho levels in mice.
✅ Plasma α-Klotho levels: Nearly doubled compared to water and whey protein groups.
✅ Brain (Hypothalamus & Amygdala): Markedly increased α-Klotho content.
✅ Kidney: Strongest α-Klotho increase—vital since kidneys produce ~80% of circulating α-Klotho.
8-day test | 60 μg/ml whey protein in water | Measured via ELISA
This research highlights MAF’s powerful potential in supporting anti-aging and longevity.
Do you feel… Aging catching up faster than you’d like?

Low energy, fatigue, or waning vitality as years go by
Declining immune strength or susceptibility to frequent illness
Concern about accelerated cellular aging, chronic stress, or oxidative damage
Worry you’ll lose your youthful vigor and longevity potential
You’re not imagining it — our bodies change at a cellular level over time. But what if you could influence that process?
A Science-Driven Approach to Rejuvenation: Dietary MAF
Developed by experts
Developed by our team of Japanese doctors at Saisei,
together with leading Japanese scientists,
Dietary MAF is a natural, food-based formulation created
to support immune resilience, cellular rejuvenation, and overall well-being.

Developed in collaboration with Japanese scientists and medical experts.

Rooted in Research. Proven in Practice.
Our formulation is supported by peer-reviewed scientific studies.
Below are some of the key research papers behind our approach.
nutrients
frontiers
Springer Nature
ScienceDirect
Scientific Reports
Globally patented formulation.



What You Stand to Gain
-
Renewed energy and vitality — feel younger, fresher, more alive
-
Enhanced immune resilience — better natural defense, fewer illnesses
-
Cellular-level rejuvenation — support DNA & gene health for long-term youth
-
Long-term well-being — invest in healthy aging, not just quick fixes
-
Confidence in aging — age gracefully with robust health
Because real longevity starts from within — at the cellular level.
How It Works
STEP 1
Clinically Studied Daily Intake
Take 2 capsules of MAF Triple daily (the dosage used in the Phase 2 clinical trial) —
1 in the morning and 1 at night, preferably on an empty stomach.
STEP 2
Cellular & Immune Support
Support cellular vitality, immune balance, and biological resilience over time.
STEP 3
Consistent Longevity Support
Continue daily use to support long-term healthy aging and overall well-being.
*Dosage based on the protocol used in the Phase 2 clinical trial.
How It Works
*Dosage based on the protocol used in the Phase 2 clinical trial.
Anti-aging and longevity
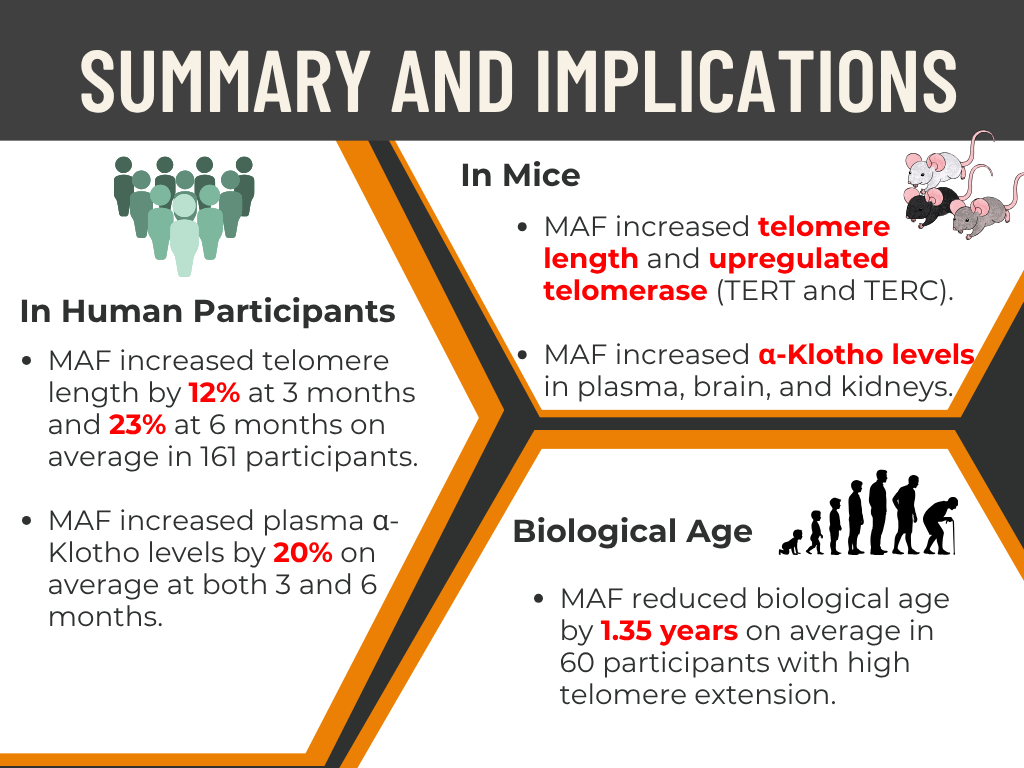
Experience the groundbreaking innovation in anti-aging science with Dietary MAF—the only clinically proven solution to extend telomeres by an extraordinary 23% within just six months. Carefully developed by Saisei Pharma, these products are designed not only to enhance your health and appearance but to help you rejuvenate your immune system, look up to 20 years younger, and live vibrantly well past a century.
At Saisei Mirai, we believe aging is not just a natural process, but a treatable condition. Through immune rejuvenation, telomere preservation, and Klotho gene activation, we are pioneering a new approach to healthy longevity.
Dietary MAF harnesses cutting-edge science to challenge the inevitability of aging, fostering a life filled with vitality and resilience. Its efficacy is underscored by detailed analyses, including studies on the impact of MAF capsules on telomere length, which have demonstrated remarkable outcomes.

Featured in the Prestigious Journal Nature
We are honored to be featured in the internationally renowned scientific journal Nature.
Our advertisement feature, titled Could immune proteins have anti-ageing benefits?, was published on March 13, 2025, as part of Focal Point on Ageing in Japan — a special editorial spotlight exploring scientific innovation in the field of aging.
Nature is one of the most respected peer-reviewed journals in the world, known for publishing groundbreaking research in science and technology.

Telomere Research by Saisei Mirai Featured in Science Featured
We are pleased to share that Dr. Toshio Inui, CEO of Saisei Mirai Clinics Group, has been featured in the respected international science publication Science Featured for his pioneering research on telomere lengthening. The article, titled Oral intake of degalactosylated whey protein increases peripheral blood telomere length in young and aged mice, was published on April 16, 2025. This recognition highlights our ongoing commitment to advancing evidence-based innovation in telomere biology, longevity science, and immune health.

Advancements in Dietary MAF: Unveiling Enhanced Rejuvenation Effects
Dietary MAF, while it still supports the immune system, shows particularly strong rejuvenation effects. In mouse studies, MAF has been shown to rejuvenate the immune system, promote telomere elongation, and increase the expression of telomerase (TERT and TERC), helping subjects regain a more youthful physiological state. Additionally, it enhanced the expression of the Klotho gene.
Therefore, we believe that MAF restores youthful immune power through mechanisms beyond traditional immune support—primarily by influencing telomeres, telomerase (TERT and TERC), and the Klotho gene. We hypothesize that telomerase activation contributes to telomere elongation, ultimately supporting longevity.
Manufactured in Japan with global patents
Unlocking the Secrets of Telomeres & Aging
Ever wondered why we age? The answer lies in telomeres —the protective caps at the ends of our chromosomes.
Degalactosylated Whey Protein (D-WP) Study – Effects on Telomere Length in Mice
A separate experimental study examined the effects of D-WP on telomere length and telomerase gene expression in young and aged mice.
📌 Findings:
✔ D-WP increased telomere length by 154% in young mice, while regular whey protein (WP) showed only a 121% increase.
✔ D-WP significantly upregulated telomerase reverse transcriptase (TERT) and telomerase RNA component (TERC) gene expression in peripheral blood.
✔ In aged mice, D-WP restored telomere length to youthful levels.
📌 Implication: D-WP, a key component in MAF Triple, enhances telomere maintenance, supporting longevity and cellular health.
🔗 Access the full study: Scientific Reports (2024)

Phase 2 Clinical Trial in Japan
📌 Study Objective: Evaluating the efficacy of Dietary MAF for rejuvenation and longevity
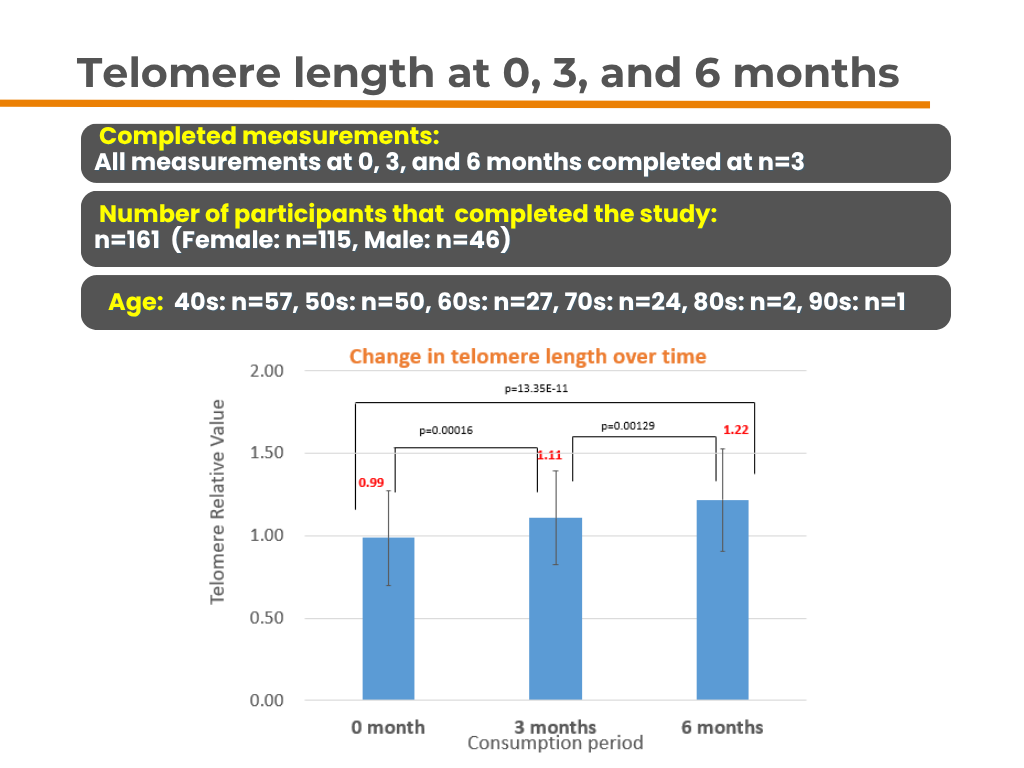
📌Number of Participants: 161
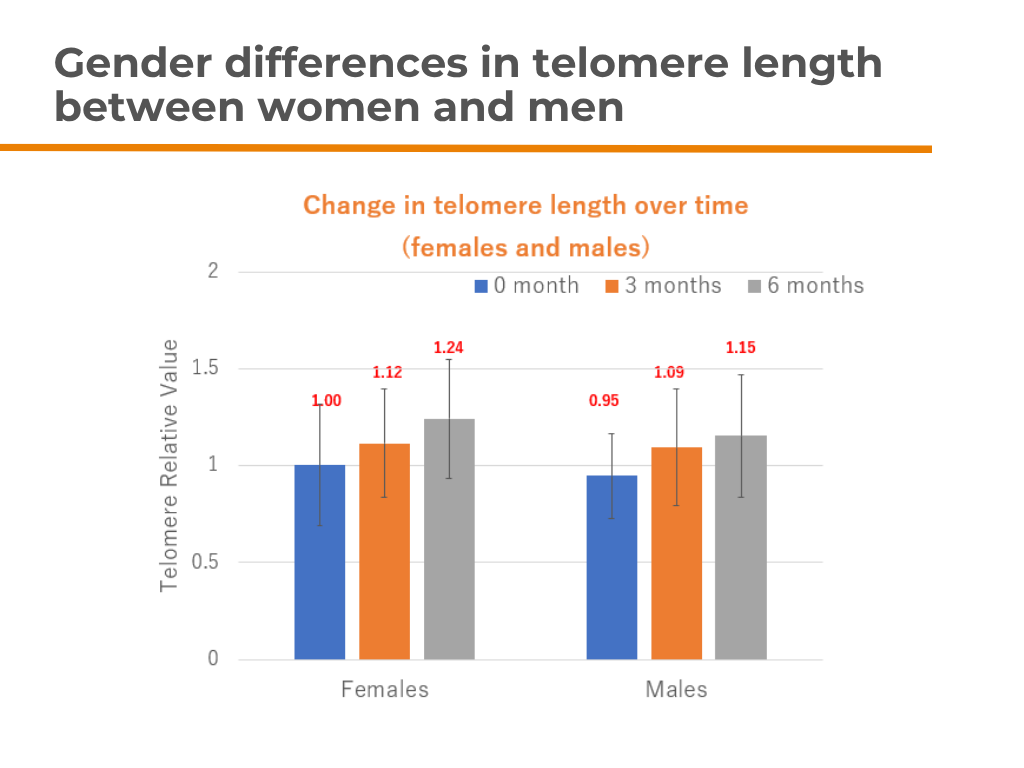
📌Results: ✔ Telomere length increased by 12% at 3 months
✔ Telomere length increased by 23% at 6 months
✔ Biological age reduction observed within 6 months of MAF Triple intake
🔬 Ongoing Research: A similar clinical trial is currently being conducted in Indonesia to further evaluate MAF Triple’s rejuvenation and longevity effects.

Association with Aging
A New Era of Dietary Rejuvenation: Phase 2 Clinical Trial of Dietary MAF in Japan
The desire to live longer—and live well—is universal. Yet as...
Read MoreSaisei Medical Conference 2025
📍 Osaka International Convention Centre, 📅 Saturday, November 8, 2025 |...
Read MoreSaisei Mirai Featured in Science Featured – Telomere Research Highlighted!
Dr. Inui’s Research on Telomere Length Featured in Science Featured...
Read MoreThe Anti-Aging Conference 2025 has successfully concluded— the recordings are now available!
Thank you for joining us — the recordings are now...
Read MoreDietary MAF Series
Dietary MAF (Macrophage Activating Factor) Series boosts immunity by activating the immune cells without any side effects. Stay young and healthy by taking these anti-aging supplements.

Free from
Wheat, soy, egg, rice, gluten, starch, sugar*, hormones, antibiotics, preservatives, artificial sweeteners, artificial colors.
*Sugar is contained in M-Lollies
Topical and Oral
MAF is available in both topical and oral supplement forms. The 4 types of MAF products allows MAF to be absorbed from the mouth, intestines and the skin. The MAF Series is made exclusively with natural ingredients.

Uniqueness
MAF was developed using our own unique patented method. MAF helps boost the immune system by activating macrophages without any side effects.

Factory in Japan
MAF Series is produced in our Saisei Mirai GMP factory in Moriguchi, Osaka. The factory is certified by the Ministry of Health, Labor and Welfare of Japan.
Boost your immunity with MAF Series
MAF = Macrophage Activating Factor
Our Dietary MAF boosts your immunity by activating the immune cells without any side effects and help you stay young and healthy. The MAF Series is made exclusively with natural ingredients and produced in Osaka, Japan.
How MAF Triple Enhances Macrophage Activation for Optimal Immune Defense
Your immune system relies on macrophages—specialized cells that identify and eliminate harmful pathogens. However, due to aging, stress, or environmental factors, macrophages can become sluggish and ineffective. By promoting this natural immune cycle, MAF Triple supports overall health, resilience, and rapid immune response.
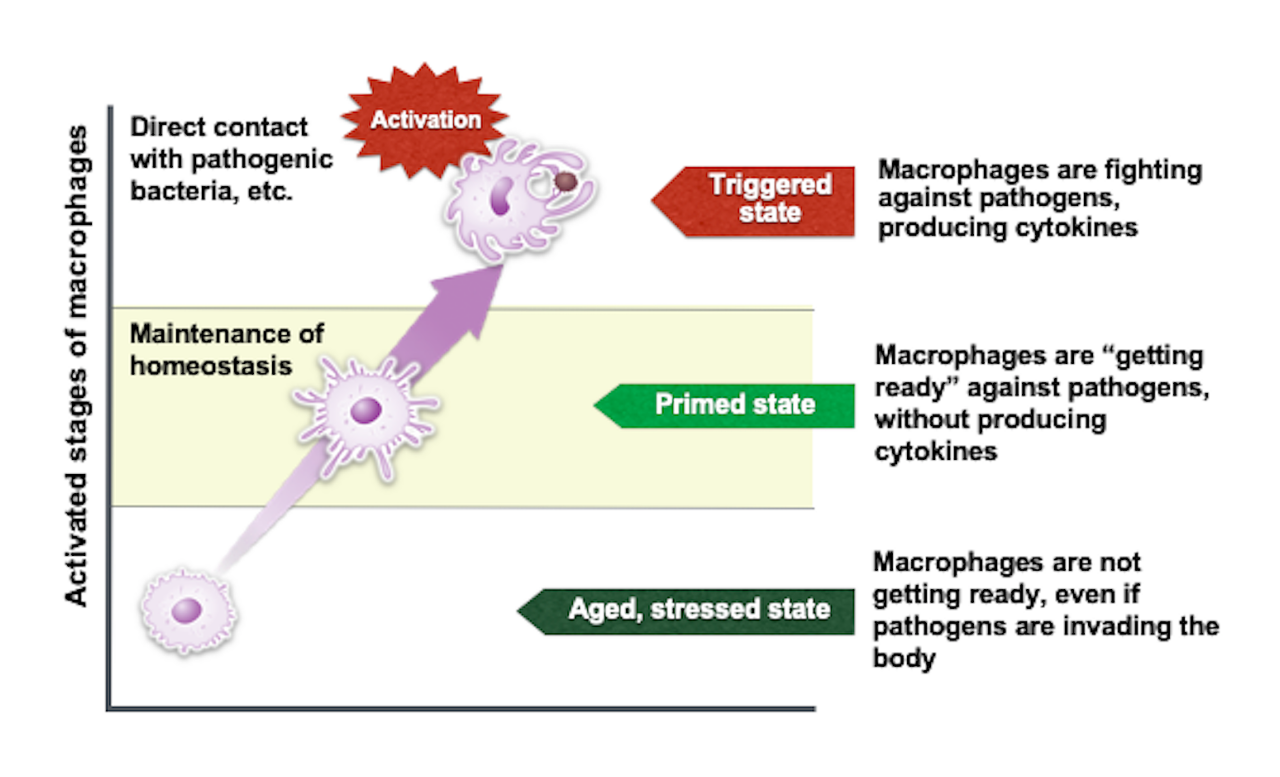
MAF Triple (Macrophage Activating Factor) helps revitalize your immune function by guiding macrophages through three crucial activation stages:
Aged, Stressed State:
Inactive macrophages fail to respond to infections.
Primed State:
Macrophages start preparing to combat pathogens without producing inflammatory cytokines.
Triggered State:
Fully activated macrophages effectively fight infections by engulfing pathogens and releasing immune-boosting cytokines.
Resident macrophages are present throughout the human body,
from the brain (microglia) and eyes (intraocular macrophages) to the lungs (alveolar macrophages), liver (Kupffer cells), spleen (splenic macrophages), intestines (intestinal macrophages), bone marrow, lymph nodes, skin (Langerhans cells), and even bones (osteoclasts), where they play essential roles in immune defense, antigen presentation, tissue homeostasis, and pathogen clearance.
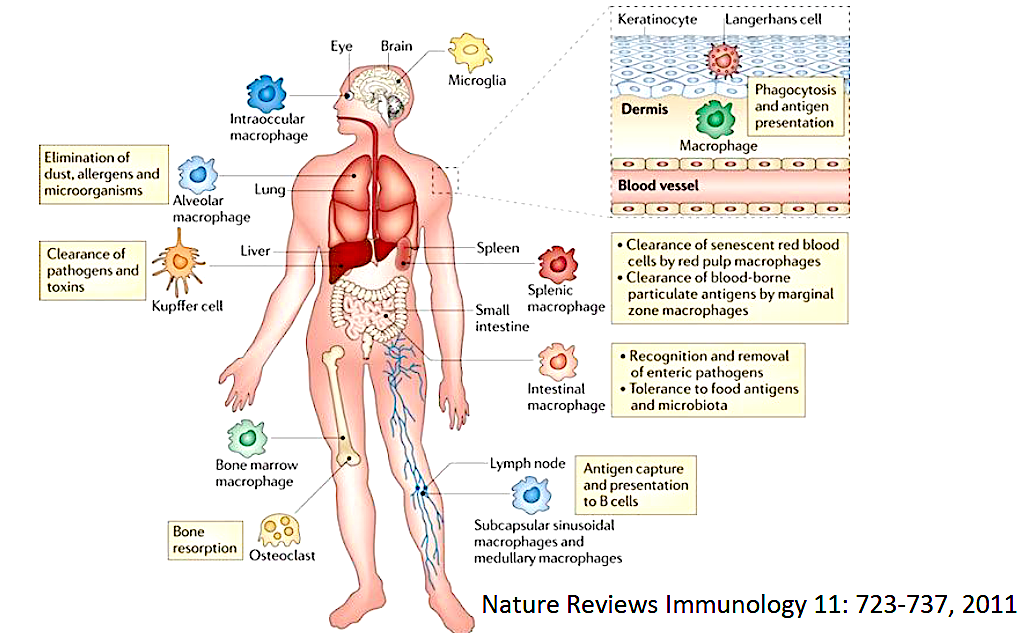
What are macrophages?
from the brain (microglia) and eyes (intraocular macrophages) to the lungs (alveolar macrophages), liver (Kupffer cells), spleen (splenic macrophages), intestines (intestinal macrophages), bone marrow, lymph nodes, skin (Langerhans cells), and even bones (osteoclasts), where they play essential roles in immune defense, antigen presentation, tissue homeostasis, and pathogen clearance.