Research Papers and Educational
Oral intake of degalactosylated whey protein increases peripheral blood telomere length in young and aged mice
The Effects of Dietary Intervention and Macrophage-Activating Factor Supplementation on Cognitive Function in Elderly Users of Outpatient Rehabilitation
Degalactosylated Whey Protein Suppresses Inflammatory Responses Induced by Lipopolysaccharide in Mice
The Effect of MAF Capsules and M Capsules on Lymphopenia and Clinical Outcomes in Non-Critical Hospitalized COVID-19 Patients.
Adjunctive use of oral MAF is associated with no disease progression or mortality in hospitalized patients with COVID-19 pneumonia: The single-arm COral-MAF1 prospective trial (Italy)
Trial Efficacy of Saisei Pharma Dietary Supplements MAF Capsules, 148 mg and M Capsules, 148 mg in Hospitalized COVID-19 Patients (SaiseiCovUKR)
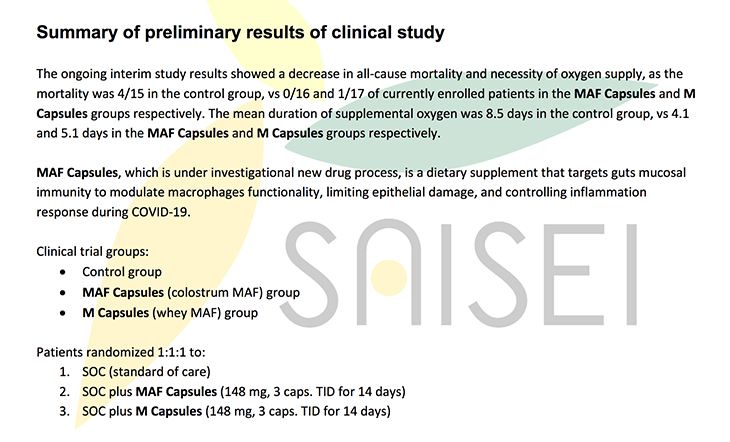
Summary of preliminary results of clinical study
In June 2020 we applied to the COVID-19 Scientific Technical Triage of the US FDA for the evaluation of the rationale to study the efficacy of MAF Capsules in COVID-19 treatment. The US FDA in PreIND 151946 meeting response recommended a small proof of concept (POC) study as the initial step prior to the large-scale trial be run. The US FDA indicated recommendations including the major study endpoints addressing the investigation of MAF Capsules efficacy as a potential new drug was implemented in the proposed study design.
Lucrezia Spaderaa, Maria Spaderab
The hypothesis: Based on the aforementioned findings and on documented analogies between SARS-CoV-2 and HIV, we hypothesized that the reduced conversion activity of the Gc protein (human group-specific component (Gc)) into the macrophage activating factor (MAF) could have a key role in the dysregulate immune response induced by SARS-CoV-2, just like for HIV infected patients. If this hypothesis is correct, it might help to set a valid strategy of immunotherapy also based on an off-label use of GcMAF in critically ill COVID-19 patients.
Abstract: Recently, immunotherapy has emerged as a new and appealing strategy for cancer treatment and various other acute and chronic diseases. Essential components of the natural immune system—phagocytic cells called macrophages—multiply in response to an infection in the body. The use of a macrophage activating therapy, such as macrophage activating factor (MAF), has extensive applications for treating numerous diseases by activating the natural macrophages of the body to stimulate the immune system. The aim of this review is to provide insight into the features and clinical efficacy of a new type of macrophage-activating factor derived from colostrum, called colostrum MAF.